The power of RDN, the experience of 360° ultrasound.
Paradise® Ultrasound
Renal Denervation System
With 360° ablation technology and exclusive features designed to ensure a safe and effective procedure, the Paradise Ultrasound Renal Denervation (uRDN) system is designed to offer a proven therapy option to lower your patients’ blood pressure.1-2
Hypertension remains a leading cause of cardiovascular issues, including heart failure, stroke, coronary heart disease, and more. And control rates around the globe are declining.
120 million*
Adults in the USA
1 in 5*
Controlled
The Paradise uRDN system treats hypertension by ablating the nerves around the renal arteries, disrupting the overactive sympathetic nerves that can cause hypertension.
See if Paradise uRDN is right for your patient.
* Estimated Hypertension Prevalence, Treatment, and Control Among U.S. Adults | Mission Hearts® (hss.gov)
** World Health Organization; https://www.who.int/news-room/fact-sheets/detail/hypertension accessed on 7/09/2023
Maximize performance.
With exclusive features designed to ensure a safe and eff ective procedure, the Paradise uRDN system off ers a proven therapy option to lower your patients’ blood pressure.1-3

The SonoWave 360™ transducer.
SonoWave 360™ transducer maximizes treatment zone with circumferential ablation, emitting ultrasound in 360° with no tissue contact.
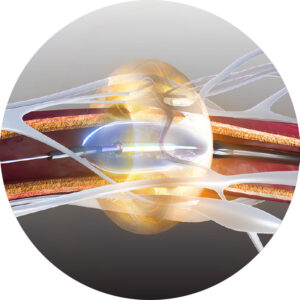
2-3 ablations per artery, 7 seconds each.
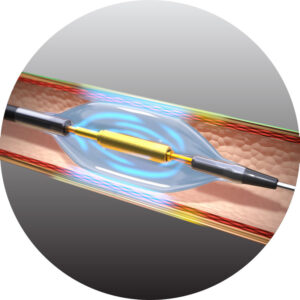
Precision without contact. Protection through cooling.
The Paradise uRDN system treats hypertension by ablating the nerves around the renal arteries, disrupting the overactive sympathetic nerves that can cause hypertension. See if Paradise uRDN is right for your patient.
Hear experts share their experience with the Paradise uRDN system.
Deliver lasting outcomes.
Off Medication
On Medication
Uncontrolled Hypertension
Resistant Hypertension
–10.4 mmHg
Reduction in office systolic BP from baseline at 2 months in combined results from RADIANCE RCTs8
3 years
RADIANCE-HTN SOLO:
Office SBP reduction -17.7 mmHg at 36 months4
RADIANCE-HTN TRIO:
Office SBP reduction -17.3 mmHg* at 36 months9,**
8 years
*in patient population with baseline office SBP ≥ 150 mmHG
**Baseline measurement occurred following 4-week standardization on a 3-drug single-pill
***Linear mixed-effect models adjusted for medications. Potential procedure-rated AEs including pain, vascular access site complications, and vasospasm are most common. Individual results may vary. See full Important Safety Information here.
Unlock efficiency.
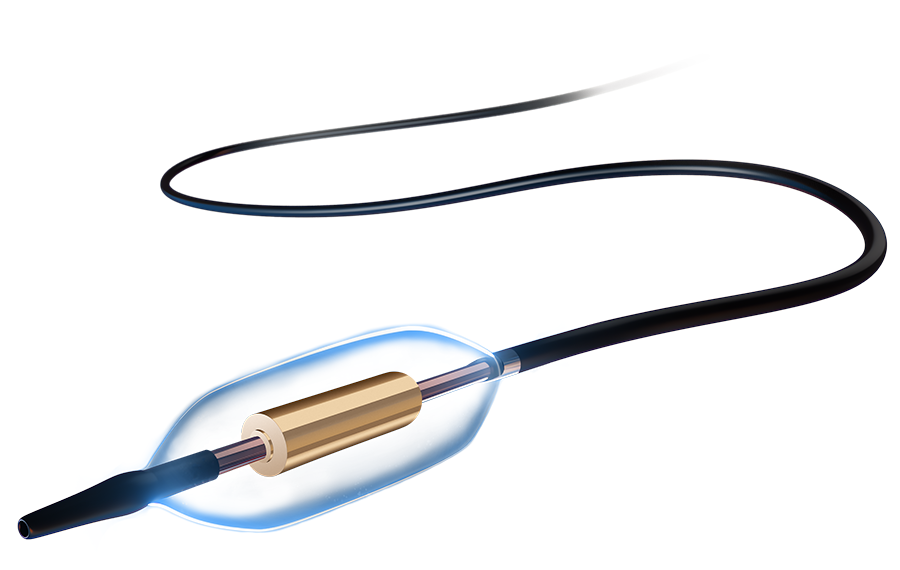
Engineered with shorter ablation time and fewer ablations in total, as well as less fluoroscopy and less contrast volume11, delivering an experience that’s predictable for your device use, and minimizes discomfort and risk for your patients.
The Paradise uRDN system was built to set a new standard in blood pressure control – a high-performing, ultrasound renal denervation system that delivers a premium experience.
- Data on file.
- Pathak et al. EuroIntervention. 2015 Aug;11(4):477-84
- Data on file. Recor Medical; Anatomies may vary, see IFU.
- Rader et al. EuroIntervention 2022;18:e677-e685.
- Bloch et al. AHA 2023 (n=49)
- Bloch et al. TCT 2023 (n=64)
- V. Zeijen, ACHIEVE Study update. TCT 2023. (n=27). Open-label follow-up data (not part of PMA pivotal data)
- Kirtane et al. JAMA Cardiol. 2023;8(5):464-473
- Bloch et al. Hypertens Res. 2024.
- Zeijen VJM Clini Res Cardiol . 2024. p-value ≥ 0.001 for all other changes compared to baseline.
- Fengler et al. Circulation. 2019 Jan 29;139(5):590-600.
- Renal arteries diameter <3 mm and >8mm
- Renal artery Fibromuscular disease (FMD)
- Stented renal artery
- Renal artery aneurysm
- Renal artery diameter stenosis >30%
- Pregnancy
- Presence of abnormal kidney (or secreting adrenal) tumors
- Iliac/femoral artery stenosis precluding insertion of the catheter
- Failure to use the recommended balloon size may result in renal artery stenosis, dissection, perforation, aneurysm, significant vasospasm requiring intervention, ablation of unintended tissues or structures, and/or no ablation of target tissue achieved.
- Energy emission in an unintended location may result in unintended tissue damage.
- Do not move the Paradise Catheter during sonication.
- Do not sonicate in renal artery at locations with visible plaque.
- Do not deliver sonications in an overlapping arterial target zone.
- Patients with known allergy to contrast medium may be at increased risk of hypersensitivity reactions.
- Only use specified coolant (Sterile water) for fluid supply. DO NOT USE SALINE.
- Avoid multiple balloon inflations to achieve apposition of the balloon to the renal artery wall; multiple balloon inflations may result in increased vessel trauma.
- The Paradise Catheter is for single use only. Do not resterilize or reuse. Reuse, reprocessing, or resterilization will compromise device integrity which may result in patient injury, illness, or death.
- Do not touch the Paradise Catheter balloon during sonication, as it may result in serious injury.
- The Paradise System may interfere with or adversely affect the operation of cardiac pacemakers or other active implants, unless proper precautions have been taken or managed per the manufacturer’s instructions. When in doubt regarding possible hazards, seek qualified advice and/or consult with the manufacturer(s) prior to initiating a procedure. The Paradise Catheter is a Type CF, defibrillation-proof Applied Part.