Clinical Evidence
Paradise® Ultrasound Renal Denervation is designed to set a new standard in hypertension treatment
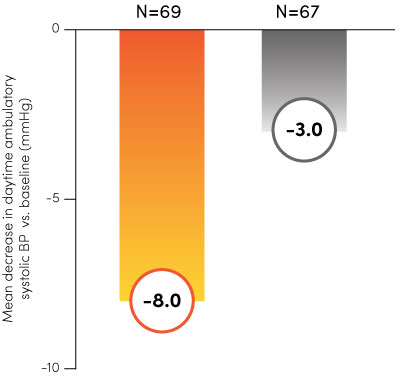
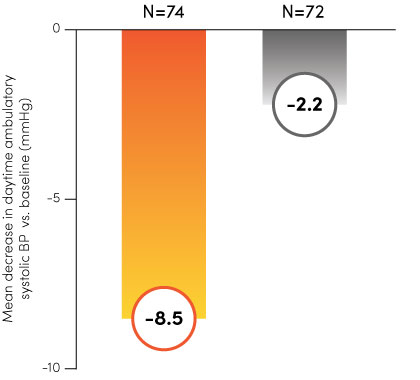
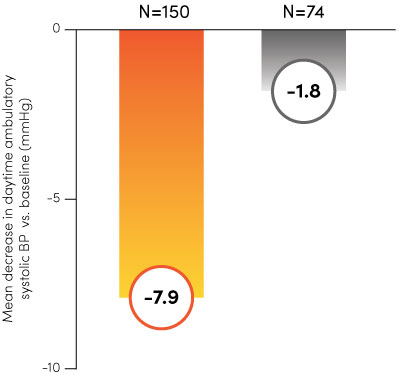
Paradise Ultrasound Renal Denervation (uRDN) has been tested and proven effective across multiple clinical trials, including Recor’s RADIANCE™ global program and the ACHIEVE study. With statistically significant and durable results1–4, the Paradise System can offer a new, clinically meaningful therapy option for you and your patients.
Drive to blood pressure goal.
-10.4
mmHg
Reduction in office systolic blood pressure from baseline at 2 months in radiance pooled randomized controlled trial.4
-17.7
mmHg
works
hours
Triple-tested, clinically validated.
The RADIANCE global program.
Paradise uRDN
Sham
Deliver lasting outcomes.
3
years
8
years
>10 mmHg blood pressure reduction. Less ablation time.8,9
Randomized head-to-head comparison of Ultrasound RDN and Radiofrequency RDN
With short 7-second bursts of ultrasound energy and 2–3 treatments in each main renal artery**11, Paradise Ultrasound RDN delivers meaningful reductions in blood pressure while shortening fluoroscopy time and reducing contrast load.9,10
*RADIOSOUND-HTN study enrollment period: 2015-2018. Antihypertensive medications remained stable through 6 months unless indicated by symptomatic hypo- or hypertension.
**Treatment site may vary with anatomy. Please refer to Paradise Catheter IFU for treatment strategy.
Resources
Primary endpoint publications
Reference
- Azizi M. et al., JAMA. 2023;329:651-661.
- Azizi M. et al., Lancet. 2018;391:2335-2345.
- Azizi M. et al., Lancet. 2021;397:2476-2486.
- Kirtane et al. JAMA Cardiol. 2023;8(5):464-473.
- Rader et al. EuroIntervention.2022;18:e677-e685.
- Bloch et al. Hypertens Res. 2024.
- Kirtane et al. TCT2022
- V. Zeijen, ACHIEVE Study update. TCT 2023
- Fengler et al., Circulation. 2019;139:590-600.
- Fengler et al., JACC Cardivasc Interv. 2023;16:367-369.
- Data on file.
Important Safety Information Rx Only. Brief Summary – Prior to use, please reference the Instructions for Use
Indications for Use The Paradise Ultrasound Renal Denervation System (Paradise System) is indicated to reduce blood pressure
as an adjunctive treatment in hypertension patients in whom lifestyle modifications and antihypertensive medications do not adequately control blood pressure.
Contraindications The Paradise Catheter is contraindicated in any of the following: Renal arteries diameter
- <3 mm and >8 mm
- Renal artery Fibromuscular disease (FMD)
- Stented renal artery
- Renal artery aneurysm
- Renal artery diameter stenosis >30%
- Pregnancy
- Presence of abnormal kidney (or secreting adrenal) tumors
- Iliac/femoral artery stenosis precluding insertion of the catheter
Warnings
- Failure to use the recommended balloon size may result in renal artery stenosis, dissection, perforation, aneurysm, significant vasospasm requiring intervention, ablation of unintended tissues or structures, and/or no ablation of target
tissue achieved. - Energy emission in an unintended location may result in unintended tissue damage.
- Do not move the Paradise Catheter during sonication.
- Do not sonicate in renal artery at locations with visible plaque.
- Do not deliver sonications in an overlapping arterial target zone.
Precautions
- Patients with known allergy to contrast medium may be at increased risk of hypersensitivity reactions.
- Only use specified coolant (Sterile water) for fluid supply. DO NOT USE SALINE.
- Avoid multiple balloon inflations to achieve apposition of the balloon to the renal artery wall; multiple balloon inflations may result in increased vessel trauma.
- The Paradise Catheter is for single use only. Do not resterilize or reuse. Reuse, reprocessing, or resterilization will compromise device integrity which may result in patient injury, illness, or death.
- Do not touch the Paradise Catheter balloon during sonication, as it may result in serious injury.
- The Paradise System may interfere with or adversely affect the operation of cardiac pacemakers or other active implants, unless proper precautions have been taken or managed per the manufacturer’s instructions. When in doubt regarding possible hazards, seek qualified advice and/or consult with the manufacturer(s) prior to initiating a procedure. The Paradise Catheter is a Type CF, defibrillation-proof Applied Part.
Potential risks of renal denervation procedure/response to treatment Ablation or thermal injury to vessel, adjacent tissue or other structures, Acute kidney injury, Angina, Anxiety, Arrhythmia, Atrial tachycardia, Bradycardia, Gastrointestinal complications (diarrhea, nausea, vomiting), Hypotension/ Dizziness and/or Headaches, Hypertension, Hyperhidrosis, Pain (transient abdominal, lower back), Renal failure or renal insufficiency, Renal artery aneurysm or pseudoaneurysm, Renal infarction, Renal artery dissection, or perforation, Renal artery stenosis, Vasospasm, Vasovagal response, Stroke or transient ischemic event.
Potential risks of arterial catheterization procedure Allergic reaction to contrast, Arterio-enteric fistula, Arterio-venous fistula, Bleeding, Cardiopulmonary arrest, Complications related to pain and anti-anxiety medications, Death, Deep vein thrombosis, Edema, Embolism (pulmonary, renal, peripheral vasculature, plaque), Hematuria, Infection, Myocardial infarction, Pain, Vascular access site complications (pseudoaneurysm, pain, swelling, hematoma)