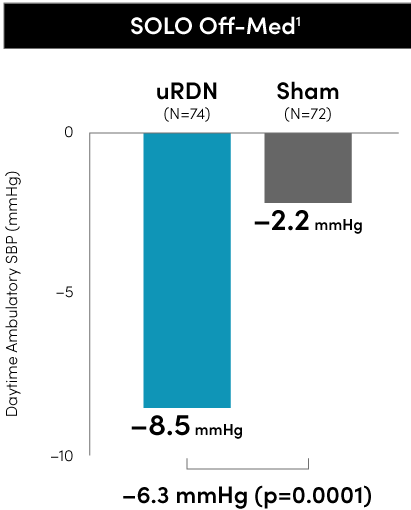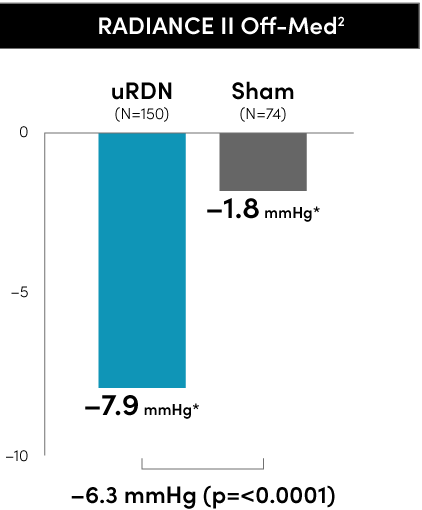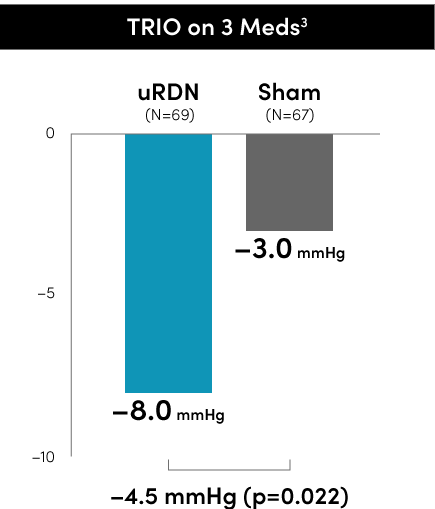Clinical Evidence
Effective. Lasting. Proven.
The Paradise® Ultrasound RDN system has been tested and proven effective across multiple clinical trials, including Recor’s RADIANCE™ global program and the ACHIEVE study. With statistically significant and durable results, the Paradise system can offer a new, clinically meaningful therapy option for you and your patients.
Met primary endpoints in
3/3
U.S. randomized controlled trials1-3
1. Azizi et al. JAMA. 2023;329(8):651-661 2. Azizi et al. Lancet. 2018 Jun 9;391(10137):2335-2345. 3. Azizi et al. Lancet. 2021 Jun 26;397(10293):2476-2486.
Endpoints met. With significance.
The RADIANCE™ global program.
Through the successful RADIANCE global program, the Paradise system became the only RDN modality to meet its primary efficacy endpoint in three separate powered, randomized controlled trials (RCTs) in the U.S., and the only RDN system to prove efficacy in resistant hypertension via a controlled trial.1-4
RADIANCE-HTN SOLO
Mild-to-moderate hypertension
RADIANCE-HTN TRIO
Resistant hypertension
RADIANCE II
Mild-to-moderate hypertension
The RADIANCE-HTN clinical trials:
- Sham controlled
- Individually powered
- Demonstrated effectiveness in lowering blood pressure at two months and beyond
The Paradise Ultrasound RDN system met the primary blood pressure reduction endpoints in all three RADIANCE global program RCTs to date.
Primary efficacy endpoint at 2 months
*individual group changes are based on observed values uRDN N=145 and Sham N=73
1. Azizi et al. Lancet. 2018 Jun 9;391(10137):2335-2345. 2. Kirtane et al. TCT2022 3. Azizi et al. Lancet. 2021 Jun 26;397(10293):2476-2486. 4. Kirtane et al. JAMA Cardiol. 2023;8(5):464-473. 5. Rader et al. EuroIntervention 2022;18:e677-e685
Ultrasound RDN was demonstrated to be safe from procedure to 36 months.5

Ultrasound RDN has consistently proven to be safe and effective across three RADIANCE randomized controlled trials studying more than 500 patients.1-4
- 1. Azizi et al. Lancet. 2018 Jun 9;391(10137):2335-2345. 2. Kirtane et al. TCT2022 3. Azizi et al. Lancet. 2021 Jun 26;397(10293):2476-2486. 4. Kirtane A. et al. JAMA Cardiol. 2023;8(5):464-473. 5. Rader et al. EuroIntervention 2022;18:e677-e685
Lasting, powerful results.
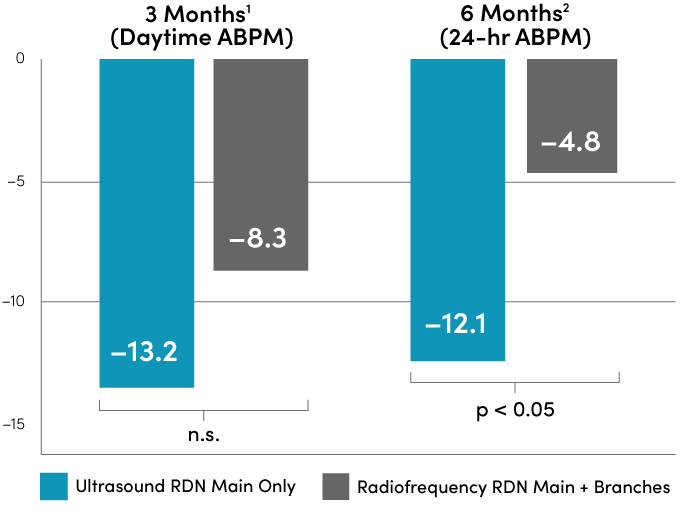
>10 mmHg BP reduction. Less ablation time.
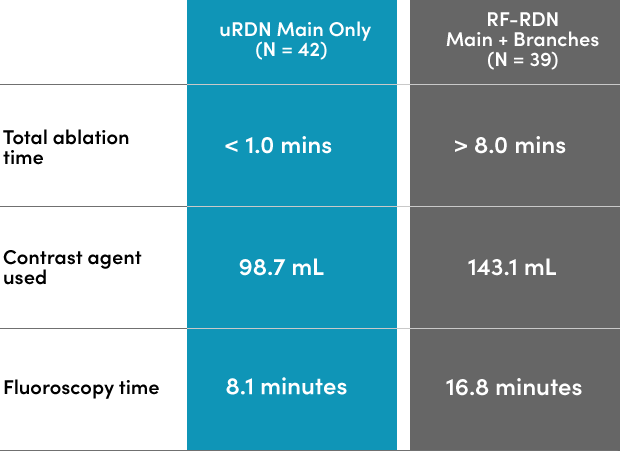
Randomized head-to-head comparison of Ultrasound RDN and Radiofrequency RDN
With short 7-second bursts of ultrasound energy and 2-3 treatments in each main renal artery, Paradise Ultrasound RDN delivers meaningful reductions in blood pressure while shortening fluoroscopy time and reducing contrast load.
*RADIOSOUND-HTN study enrollment period: 2015-2018. Antihypertensive medications remained stable through 6 months unless indicated by symptomatic hypo- or hypertension.
1. Fengler et al. Circulation. 2019 Jan 29;139(5):590-600. 2. Fengler et al. JACC: Cardiovascular Interventions, 2023, Feb 13 :359-369