Intended for Healthcare Professionals Only
A new, proven treatment to reduce blood pressure when lifestyle and medication management aren’t enough.*
Paradise® Ultrasound
Renal Denervation (uRDN)
>10mmHg
OFFICE BP REDUCTION
in Radiance RCT combined results at 2 months1
in Radiance RCT combined results at 2 months1
Clinically proven safe and effective.
Met all primary
endpoints in
3/3
U.S. randomized
controlled trials2-4
controlled trials2-4
Blood pressure
reductions2-4
reductions2-4
Active Study
–22.1mmHg
Office BP
reductions at 8
years (n=27)5
reductions at 8
years (n=27)5
Impact to
renal function
at 2 months1
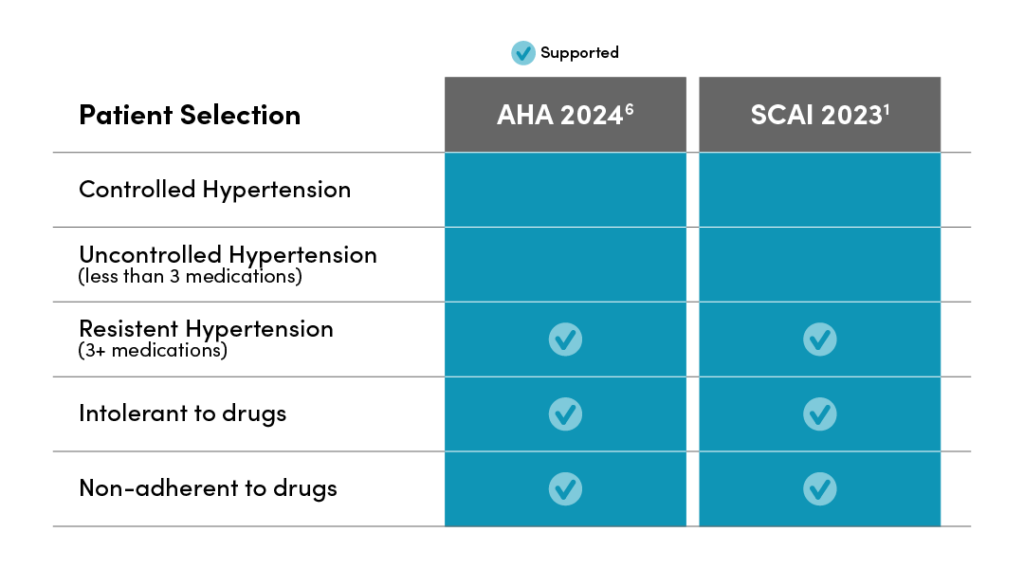
Hypertension consensus statements support the use of RDN to lower blood pressure.
Patients benefited from Paradise uRDN.

“Following my procedure with Paradise Ultrasound RDN, my blood pressure is better controlled.”
Candyce, 73 years old
Candyce, 73 years old

“Going for treatment with the Paradise Ultrasound RDN procedure was a great decision for me because now I have only one blood pressure medication. Prior to it, it was all over the board, I was taking all of those medications and my blood pressure was up and down. For me, personally, it’s a life saver.”
David, 48 years old
David, 48 years old
Actual patients treated with Paradise uRDN. Individual results may vary.
How uRDN works
- Proven procedural protection and safety.2-4
- Safe, minimally invasive procedure.
- Provides 24-hour blood pressure
reductions2-4 without the side effects of
medications.
Get more information about how Paradise Ultrasound RDN can help your patients control high blood pressure
References
- Kirtane A. et al. JAMA Cardiol. 2023;8(5):464-473.
- Azizi M. et al. Lancet. 2021 Jun 26;397(10293):2476-2486.
- Azizi M. JAMA. 2023;329(8):651-661.
- Azizi M. et al. Lancet. 2018 Jun 9;391(10137):2335-2345.
- Zeijen et al. ACHIEVE Study, TCT 2023.
- Cluett L. et al. Hypertension. 2024;81:e00–e00.
- Swaminathan et al. Journal of the Society for Cardiovascular Angiography & Interventions, published ahead of print on 21 August, 2023.
* Rx Only. Brief Summary – Prior to use, please reference the Instructions for Use
Indications for Use
The Paradise Ultrasound Renal Denervation System (Paradise System) is indicated to reduce blood
pressure as an adjunctive treatment in hypertension patients in whom lifestyle modifications and
antihypertensive medications do not adequately control blood pressure.
Contraindications
The Paradise Catheter is contraindicated in any of the following:
- Renal arteries diameter <3 mm and >8mm
- Renal artery Fibromuscular disease (FMD)
- Stented renal artery
- Renal artery aneurysm
- Renal artery diameter stenosis >30%
- Pregnancy
- Presence of abnormal kidney (or secreting adrenal) tumors
- Iliac/femoral artery stenosis precluding insertion of the catheter Warnings
- Failure to use the recommended balloon size may result in renal artery stenosis, dissection, perforation, aneurysm, significant vasospasm requiring intervention, ablation of unintended tissues or structures, and/or no ablation of target tissue achieved.
- Energy emission in an unintended location may result in unintended tissue damage.
- Do not move the Paradise Catheter during sonication.
- Do not sonicate in renal artery at locations with visible plaque.
- Do not deliver sonications in an overlapping arterial target zone
Precautions
- Patients with known allergy to contrast medium may be at increased risk of hypersensitivity reactions.
- Only use specified coolant (Sterile water) for fluid supply. DO NOT USE SALINE.
- Avoid multiple balloon inflations to achieve apposition of the balloon to the renal artery wall; multiple balloon inflations may result in increased vessel trauma.
- The Paradise Catheter is for single use only. Do not resterilize or reuse. Reuse, reprocessing, or resterilization will compromise device integrity which may result in patient injury, illness, or death.
- Do not touch the Paradise Catheter balloon during sonication, as it may result in serious injury.
- The Paradise System may interfere with or adversely affect the operation of cardiac pacemakers or other active implants, unless proper precautions have been taken or managed per the manufacturer’s instructions. When in doubt regarding possible hazards, seek qualified advice and/or consult with the manufacturer(s) prior to initiating a procedure. The Paradise Catheter is a Type CF, defibrillation-proof Applied Part.Potential risks of renal denervation procedure/response to treatment Ablation or thermal injury to vessel, adjacent tissue or other structures, Acute kidney injury, Angina, Anxiety, Arrhythmia, Atrial tachycardia, Bradycardia, Gastrointestinal complications (diarrhea, nausea, vomiting), Hypotension/ Dizziness and/or Headaches, Hypertension, Hyperhidrosis, Pain (transient abdominal, lower back), Renal failure or renal insufficiency, Renal artery aneurysm or pseudoaneurysm, Renal infarction, Renal artery dissection, or perforation, Renal artery stenosis, Vasospasm, Vasovagal response, Stroke or transient ischemic event Potential risks of arterial catheterization procedure Allergic reaction to contrast, Arterio-enteric fistula, Arterio-venous fistula, Bleeding, Cardiopulmonary arrest, Complications related to pain and anti-anxiety medications, Death, Deep vein thrombosis, Edema, Embolism (pulmonary, renal, peripheral vasculature, plaque), Hematuria, Infection, Myocardial infarction, Pain, Vascular access site complications (pseudoaneurysm, pain, swelling, hematoma)